Sonographic Pulse Check in Cardiac Arrest
Noah J. Hillerbrand, MD
Virginia Commonwealth University
Stephen M. Miller, DO
Virginia Commonwealth University
Jordan Tozer, MD
Virginia Commonwealth University
Case
A 78-year-old male with known atherosclerotic heart disease was found unresponsive at home. Paramedics arrived to find him in pulseless electrical activity (PEA). CPR was initiated, he was intubated and given 1 mg epinephrine via intravenous line. While en route to the hospital, there was a return of spontaneous circulation. Within minutes of arrival in the ED, there is disagreement regarding whether a pulse was palpable. His monitor shows a narrow complex rhythm. What is the most efficient method to determine if a pulse is present?
Background
The use of point-of-care ultrasound in cardiac arrest has grown in recent years. Its utility ranges from identification of the etiology of arrest, assessing effectiveness of cardiopulmonary resuscitation (CPR) and in determining return of spontaneous circulation (ROSC). Both the American Heart Association (AHA) and European Resuscitation Councils’ (ERC) most recent guidelines recommend the use of manual palpation to identify successful ROSC and the presence of a pulse.1,2 However, numerous studies have demonstrated the inaccuracy with which both trained healthcare professionals and lay-people are able to palpate a central pulse; with reported accuracy as low as 54%.3,4 While the gold standard for determining ROSC remains the use of invasive arterial monitoring, achieving this requires the availability of a proceduralist capable of performing this procedure, in many cases while CPR is ongoing, and the appropriate monitoring equipment at bedside. In many clinical areas, this is not feasible and the reliance on manual pulse detection remains standard. In most acute care settings, point-of-care ultrasound (POCUS) has become more mobile and readily available, and their use may increase the speed and accuracy of identifying a pulse. This has led to the increased interest in the role of POCUS for pulse detection as an alternative to manual palpation, especially in situations in which more invasive monitoring is unavailable.5 This summary aims to review common methods used in sonographic pulse checks as well as review areas of future interest.
Site
As with manual palpation for pulse detection, most research studies and clinical use of ultrasound for pulse detection focuses on the central vessels, specifically the common femoral artery and common carotid artery. No specific study has compared the superiority of the two sites to each other. Both the common femoral artery and the common carotid artery benefit from being relatively easily accessible and do not require interruption of chest compressions to access the site. Patient factors such as ongoing airway management, ongoing vascular access procedures, traumatic injuries, cervical spine immobilization, body habitus, and operator comfort will affect site selection. For the purposes of this review, both sites are considered viable and equivalent for their use in a sonographic pulse check.
Ultrasound Technique
Three techniques have been described for POCUS pulse checks. These are compression, color flow doppler and pulsed wave doppler. For all methods, it is advisable to use time during ongoing chest compressions to obtain an optimized view of either of the arteries (with appropriate image depth, gain, doppler settings) to maximize the chances of obtaining the required view and assessment during a check. If feasible, video or screen recording should be performed so the clip can be further analyzed if needed while compressions are resumed.6
Compression
Perhaps the most accessible version of sonographic pulse check is the compression method. Using standard grey scale (B-mode) imaging, the operator uses the ultrasound probe to compress either the carotid artery or femoral artery until the accompanying major vein is completely collapsed, termed POCUS femoral artery compression (POCUS-FAC) or carotid artery compression (POCUS-CAC). In cases where ROSC has been achieved, the artery will not completely collapse and should display pulsatility.
Previous research has shown POCUS-CAC to be faster in determining the presence of a pulse than manual palpation.7–9 In many studies, the time taken to teach compression pulse check protocol to practicing health care providers was minimal, about 10 to 20 minutes. Kang et al. found compression pulse checks took less than half the time of manual palpation across a total of 155 pulse checks in 25 cardiac arrest patients.7 Compression pulse checks never exceeded the 10 second maximum recommended by ACLS (versus five instances with manual palpitation) and only 5 instances (3%) that exceeded 5 seconds (versus 37 with manual palpation [24%]). Average time of pulse check with compression ultrasound was 1.62s (1.14 - 2.14) compared to 3.50s (2.99 - 4.99) with manual palpation.7 Utilizing identical POCUS-CAC protocol as Kang , Kanter et al. studied an additional 496 pulse checks across 41 patients with very similar results.8 This study also investigated the role of POCUS-CAC at 30 second intervals during ongoing compression and noted success in early identification of ROSC during compressions (detecting 52.9% of cases of ROSC with ongoing compressions).8
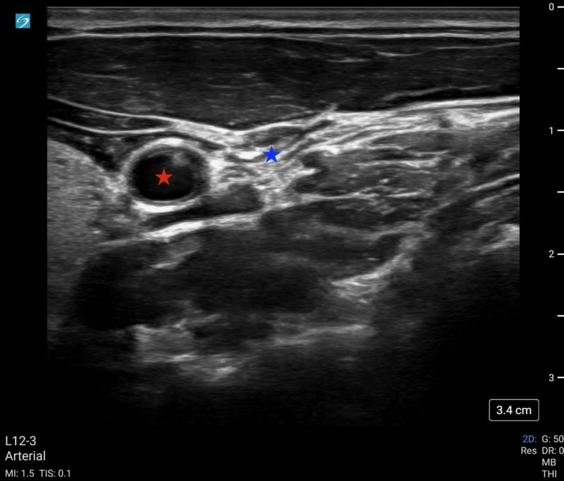 Image 1: POCUS-CAC with a fully compressed IJ (blue star) without compression of the common carotid artery (red star).
Image 1: POCUS-CAC with a fully compressed IJ (blue star) without compression of the common carotid artery (red star).
Video 1: POCUS-CAC showing complete collapse of the IJ with pulsatility of the adjacent carotid artery.
Color
Color doppler ultrasound displays the direction and velocity of flow detected in a defined area. By convention, flow towards the ultrasound is visualized as red and away as blue. To perform color doppler assessment, the operator obtains the standard 2D mode view of the artery and a color doppler box is placed over the vessel.
Literature using color flow doppler to determine ROSC is limited. Humphries et al. describes a case report on the use of color doppler in the prehospital setting to both monitor intra-arrest compression quality as well as to determine ROSC.10
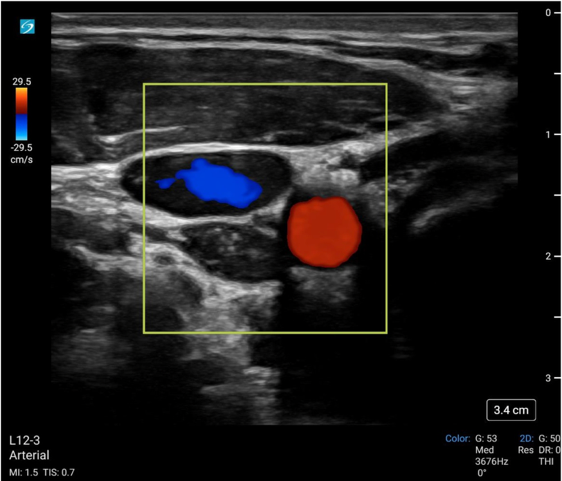 Image 2: Color flow doppler of the common carotid artery (red) and IJ vein (blue).
Image 2: Color flow doppler of the common carotid artery (red) and IJ vein (blue).
Video 2: Color flow doppler of the common carotid artery (with aliasing) during compression of the adjacent IJ vein.
Pulsed Wave Doppler
While the use of compression and color doppler aim to provide a qualitative assessment of pulsatile flow, pulsed wave doppler (PWD) of the large arteries can provide both qualitative and quantitative assessment of blood flow. Using the PWD mode, a sampling gate is centered within the artery in long axis and flow is measured. Most research thus far has focused on the peak systolic velocity (PSV) and end diastolic velocity (EDV), with more limited study of mean diastolic velocity (MDV).
The proof of concept was first published in 2015 by Adedipe et al who showed doppler measurements of carotid blood flow during cardiac arrest was feasible.11 Measurements were obtained by trained emergency physicians and investigators were able to complete a scanning protocol that included both color doppler and pulsed wave doppler measurements (PSV, EDV, MDV). Cohen et al. followed this in 2022 and showed pulsed wave doppler of the femoral artery was more accurate than manual palpation at either the femoral or carotid artery (95.3% vs. 54.0%; p < 0.001) across 213 pulse checks in 54 patients.12 A secondary outcome of that same study showed that a PSV ≥ 20 cm/s accurately detected an arterial line pulse SBP ≥ 60 mmHg with an accuracy of 91.4% and area under the receiver operating curve = 0.975. Lastly, in 2023, Haddad et al. showed the diagnostic accuracy of this same PSV cut off (≥ 20 cm/sec) for detecting ROSC with SBP of 60 mmHg was 89% (95% CI: 82%-94%) versus 59% (95% CI: 49%-68%) and 58% (95% CI: 48%-67%) for ETCO2 20 and 25 mmHg.13
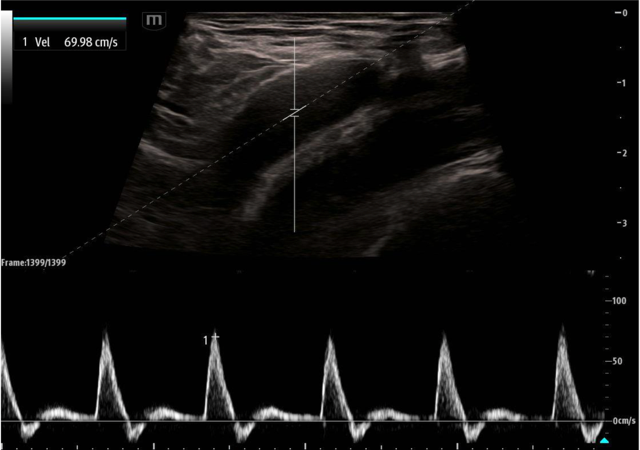 Image 3. Pulsed wave doppler of the femoral artery in long axis showing a PSV of approximately 70 cm/sec.
Image 3. Pulsed wave doppler of the femoral artery in long axis showing a PSV of approximately 70 cm/sec.
Discussion
The superior accuracy of ultrasound over manual palpation for pulse detection in cardiac arrest has been demonstrated in numerous studies with varied ultrasound techniques. There are multiple strengths to recognize from the current literature: POCUS use is widespread, available in most EDs and increasingly in the prehospital setting; compression and color ultrasound offer excellent accuracy with minimal training needed, making it easily deployable by a variety of providers; and the emerging quantitative measurements may assist a more tailored hemodynamic guided resuscitation, aiding decisions on when to terminate CPR in favor of hemodynamic support and in ceasing resuscitation efforts entirely.
Despite these strengths, the use of POCUS for pulse checks is not without limitations. There are two main areas of concern identified from the available literature. The first is the concern that the use of point of care ultrasound in cardiac arrest management can result in prolonged pulse checks, decreased chest compression fraction, and detraction from other critical tasks. Multiple early studies demonstrated longer pauses for pulse checks in cases where POCUS was used versus those with manual palpation.14–16 However, this has not been consistent throughout the research with more recent research showing both more accurate and faster pulse checks with ultrasound.7,17,18 A second concern is more specific to quantitative measurements taken when using pulse wave doppler. There is limited human evidence to guide management decisions for patients falling in the grey zone of pseudo-PEA (defined in Cohen et al. as PSV > 0 and < 20 cm/s), i.e. patients with identifiable pulsatile flow on doppler but below velocities consistent with perfusing blood pressures.12,19,20 Whether continuation of chest compressions in patients with any intrinsic pulsatility is harmful or beneficial remains unknown.21 Animal model research has suggested that chest compressions synchronized with intrinsic cardiac activity may be beneficial whereas unsynchronized compressions are harmful.22,23 However, this has not been shown to be feasible or effective in humans and the most effective resuscitation in this patient population (ie, continued chest compressions vs. medical management) remains uncertain.
Looking Forward
We anticipate the role of POCUS for pulse checks in cardiac arrest will continue to grow as will the research data supporting its use. Future areas of potential study include the use of POCUS for non-invasive intra-arrest monitoring of compression quality and hemodynamic guided resuscitation. Additionally, identification of ROSC during ongoing compressions is a promising area of study. Gaspari et al. (2023) demonstrated the ability to identify arterial doppler tracings from native cardiac-induced pulsations during active chest compressions. The study found that arterial Doppler during active CPR was 42% sensitive (95% CI, 26% to 47%) for detecting native cardiac-induced arterial pulsations during CPR compared to arterial pulsations during a pause in CPR.24 Zhao et al. (2024) describes the use of a wearable carotid doppler patch in a porcine model to monitor for ROSC as well as assess compression quality. The patch was able to provide evaluation of compression quality, with a positive correlation between doppler Vmax and compression depth as well as identify intrinsic waveforms in addition to ongoing compression-generated waveforms.25 In the future, similar continuous wearable devices may allow for CPR optimization and earlier identification of ROSC without requiring an additional operator.25-27
Case Resolution
Femoral artery POCUS showed a pulsatile, noncompressible vessel. PWD gates were placed over the vessel and demonstrated a PSV of 22 cm/s. CPR was halted and instead push-dose epinephrine was administered in 2- to 4-mcg aliquots every 30 seconds. After 15 mcg of epinephrine, the patient regained a mean arterial pressure of 65 mm Hg and palpable peripheral pulses. The patient was started on continuous norepinephrine, admitted to the ICU, and subsequently discharged home neurologically intact 16 days later. Case adapted from Simard et al. (2019).28
Summary
Achieving ROSC in a patient in cardiac arrest is the goal of cardiopulmonary resuscitation, however recognizing that transition can be difficult. There are now multiple studies that have demonstrated the poor performance of manual pulse checks. The expansion of point-of-care ultrasound in the acute care setting has led to the integration of POCUS in the evaluation and management of intra-arrest and post-arrest patients. With appropriate training, sonographic pulse checks may allow for more rapid and accurate identification of ROSC. Further study is required to determine which of these methods and in what location is most effective, as well as ideal management of patients with POCUS identified pulsatility that does not meet certain perfusion thresholds. Future research may also identify a role for POCUS in continuous intra-compression monitoring for ROSC. Incorporation of these new techniques into cardiac arrest management guidelines may be considered in the future.
References
- Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2).
- Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. 2021;161:115-151.
- Lapostolle F, Le Toumelin P, Agostinucci JM, Catineau J, Adnet F. Basic cardiac life support providers checking the carotid pulse: Performance, degree of conviction, and influencing factors. Acad Emerg Med. 2004;11(8):878-80.
- Eberle B, Dick WF, Schneider T, Wisser G, Doe&h S, Tzanova I. Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33:107-116.
- Rolston DM. Time is running out for manual pulse checks as ultrasound races past. Resuscitation. 2022;179:59-60.
- Howell K, Desai A, Martin D, Nagdev A. How To Safely Incorporate Ultrasound Into Cardiac Arrest Resuscitation. ACEP Now. September 7, 2023.
- Kang SY, Jo IJ, Lee G, et al. Point-of-care ultrasound compression of the carotid artery for pulse determination in cardiopulmonary resuscitation. Resuscitation. 2022;179:206-13.
- Kanter E, Kayalı A, Çınaroğlu OS, et al. Is It Possible to Detect Return of Spontaneous Circulation during Chest Compression? Evaluation of a Novel Method: Carotid Artery Compression Ultrasound. Diagnostics. 2024;14(19):2213.
- Chang H, Kim SG, Jo I, Yoon H. Use of Carotid Artery Compressibility Detected on Ultrasonography During Chest Compression to Validate Return of Spontaneous Circulation. Published online 2020.
- Humphries AL, White JMB, Guinn RE, Braude DA. Paramedic-Performed Carotid Artery Ultrasound Heralds Return of Spontaneous Circulation in Out-of-Hospital Cardiac Arrest: A Case Report. Prehosp Emerg Care. 2023;27(1):107-111.
- Adedipe AA, Fly DL, Schwitz SD, et al. Carotid Doppler blood flow measurement during cardiopulmonary resuscitation is feasible: A first in man study. Resuscitation. 2015;96:121-125.
- Cohen AL, Li T, Becker LB, et al. Femoral artery Doppler ultrasound is more accurate than manual palpation for pulse detection in cardiac arrest. Resuscitation. 2022;173:156-165.
- Haddad G, Margius D, Cohen AL, et al. Doppler ultrasound peak systolic velocity versus end tidal carbon dioxide during pulse checks in cardiac arrest. Resuscitation. 2023;183.
- Clattenburg EJ, Wroe PC, Gardner K, et al. Implementation of the Cardiac Arrest Sonographic Assessment (CASA) protocol for patients with cardiac arrest is associated with shorter CPR pulse checks. Resuscitation. 2018;131:69-73.
- Huis in ’t Veld MA, Allison MG, Bostick DS, et al. Ultrasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation. 2017;119:95-98.
- Lien WC, Chong KM, Chang CH, et al. Impact of Ultrasonography on Chest Compression Fraction and Survival in Patients with Out-of-hospital Cardiac Arrest. West J Emerg Med. 2023;24(2):322-330.
- Schwartz BE, Gandhi P, Najafali D, et al. Manual Palpation vs. Femoral Arterial Doppler Ultrasound for Comparison of Pulse Check Time During Cardiopulmonary Resuscitation in the Emergency Department: A Pilot Study. J Emerg Med. 2021;61(6):720-730.
- Özlü S, Bilgin S, Yamanoglu A, et al. Comparison of carotid artery ultrasound and manual method for pulse check in cardiopulmonary resuscitation. Am J Emerg Med. 2023;70:157-162.
- Rabjohns J, Quan T, Boniface K, Pourmand A. Pseudo-pulseless electrical activity in the emergency department, an evidence based approach. Am J Emerg Med. 2020;38(2):371-375.
- Hogan TS. External cardiac compression may be harmful in some scenarios of pulseless electrical activity. Med Hypotheses. 2012;79(4):445-447.
- Teran F, Centeno C, Lindqwister AL, et al. Epinephrine plus chest compressions is superior to epinephrine alone in a hypoxia-induced porcine model of pseudo-pulseless electrical activity. Resusc Plus. 2021;6.
- Marill KA, Menegazzi JJ, Koller AC, Sundermann ML, Salcido DD. Synchronized Chest Compressions for Pseudo-PEA: Proof of Concept and a Synching Algorithm. Prehosp Emerg Care. 2020;24(5):721-729.
- Paradis NA, Halperin HR, Zviman M, Barash D, Quan W, Freeman G. Coronary perfusion pressure during external chest compression in pseudo-EMD, comparison of systolic versus diastolic synchronization. Resuscitation. 2012;83(10):1287-1291.
- Gaspari RJ, Lindsay R, Dowd A, Gleeson T. Femoral Arterial Doppler Use During Active Cardiopulmonary Resuscitation. Ann Emerg Med. 2023;81(5):523-531.
- Zhao X, Wang S, Yuan W, Wu J, Li C. A new method to evaluate carotid blood flow by continuous Doppler monitoring during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Resuscitation. 2024;195.
- Faldaas BO, Nielsen EW, Storm BS, et al. Hands-free continuous carotid Doppler ultrasound for detection of the pulse during cardiac arrest in a porcine model. Resusc Plus. 2023;15. doi:10.1016/j.resplu.2023.100412
- Sharp WW, Beiser DG. Hands free pulse checks: The future of CPR. Resuscitation. 2024;195. doi:10.1016/j.resuscitation.2024.110121
- Simard RD, Unger AG, Betz M, Wu A, Chenkin J. The POCUS Pulse Check: A Case Series on a Novel Method for Determining the Presence of a Pulse Using Point-of-Care Ultrasound. J Emerg Med. 2019;56(6):674-679.