ACEP Ultrasound Safety Subcommittee Reviews New High-Level Disinfection Option
Daniel Mirsch, DO, FACEP, FPD-AEMUS
Josephine Valenzuela, MD, FPD-AEMUS
Disinfection of ultrasound probes isn’t exactly the topic that gets most emergency physicians out of bed in the morning. But for those of us who’ve spent time wrestling with policy changes, regulatory requirements, and the occasional hospital-acquired infection, it’s a subject that’s impossible to ignore. The ACEP Ultrasound Safety Subcommittee recently met to tackle this issue head-on, reviewing both the current state of probe disinfection and a new product with the potential to significantly improve emergency department (ED) workflows.
Background: Why Probe Disinfection Matters
Most of us first encountered ultrasound probe disinfection as a mandatory box to check during residency or onboarding. But infection risks associated with the use of ultrasound probes are real. Even minor missteps—like using the wrong gel or skipping a cleaning step—have resulted in infection outbreaks, including Burkholderia. Regulatory agencies such as the Occupational Safety and Health Administration (OSHA), the Food and Drug Administration (FDA), and the Joint Commission pay close attention to these issues, and it’s important that we do as well.
Ultrasound probes are classified as either noncritical (touching only intact skin) or semicritical (contacting mucous membranes). The latter—think endocavitary or transesophageal probes—require high-level disinfection (HLD) to protect against bloodborne pathogens, tuberculosis, and even spores. The tools and guidelines for this have evolved, but the operational headaches associated with disinfection remain.
“For almost all of us, high-level disinfection means sonicated hydrogen peroxide mist in a dedicated machine like a Trophon,” says incoming subcommittee chair Josephine Valenzuela, MD, emergency ultrasound director at Valleywise Health. “These machines cost tens of thousands of dollars, require biomedical engineering support to maintain and cycle the hydrogen peroxide periodically, and require staff training to use. Because of this, high-level disinfection can be a significant barrier to the use of endocavitary or transesophageal probes in an emergency department setting.”
Efforts to reduce costs can make matters worse, says Valenzuela. “Some hospitals will share this equipment between the emergency department and the radiology ultrasound department, but often that leads to disinfected probes being misplaced in the imaging department instead of being returned to the ED.”
ACEP Ultrasound Safety Subcommittee: Searching for a Better Way
At our April meeting, ACEP’s Ultrasound Safety Subcommittee set out to review current guidelines from ACEP and the American Institute of Ultrasound in Medicine (AIUM) and to get a hands-on look at new disinfection options. Sean Verschuren from Parker Laboratories demonstrated the Tristel ULT system, a chlorine dioxide-based foam designed for manual application. The FDA-cleared product promises a two-minute, manual, high-level disinfection process—no soaking, no machine, and no central processing required.
According to Verschuren, you simply apply the foam using a proprietary wipe, wait two minutes, remove residue with another wipe, and the probe is ready for use. The system is portable, compatible with a range of probe types, and doesn’t require post-disinfection leak testing. For busy EDs, this could mean less downtime and fewer workflow interruptions.
Unlike other HLD modalities, Tristel ULT does not require any capital equipment expenditures or the associated costs for utilities, maintenance, and staff training. Cost per cycle is estimated to be less than $5, which is competitive with even the least expensive manual soaking methods when all costs are accounted for.
The safety subcommittee also discussed potential barriers to implementing an HLD product like this, including the need for clear labeling systems to distinguish between clean and dirty probes, and the importance of ongoing communication with infection prevention teams.
Overall, the group’s consensus was that Tristel ULT could make high-level disinfection more accessible and less disruptive, especially for EDs looking to expand endocavitary ultrasound use.
Next Steps: Guideline Revisions for a Safer, Simpler Workflow
Given the potential impact, the subcommittee has been given the green light to revise ACEP’s guidelines on transducer disinfection ahead of schedule, formally incorporating chlorine dioxide as an acceptable high-level disinfectant option.
Ultrasound probe disinfection may never be glamorous, but it’s a critical part of safe, effective emergency care. With new options like chlorine dioxide on the horizon, we have a chance to make our workflows safer and simpler. Stay tuned for more updates as we revise our guidelines and continue the conversation.
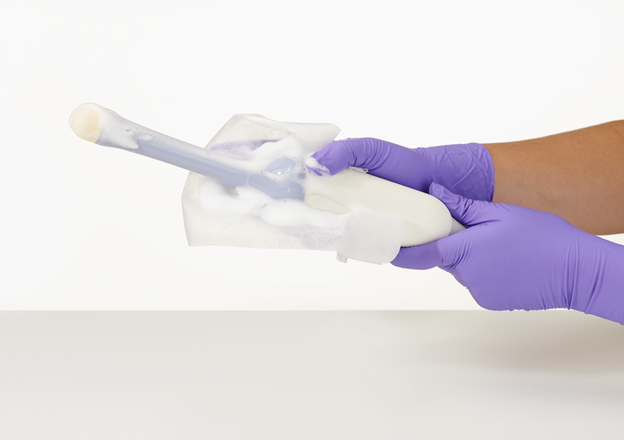
The FDA has cleared Tristel ULT, a manually applied chloride dioxide foam-and-wipe system, as a high-level disinfectant for ultrasound transducers. The photograph is not copyrighted but is courtesy of Parker Laboratories.



